Unlocking the Secrets of Limb Regeneration: How Axolotls Could Teach Humans to Regrow Limbs
Unlocking the Secrets of Limb Regeneration: How Axolotls Could Teach Humans to Regrow Limbs
Introduction: The Mystery of Regrowth
Imagine losing a limb and watching it grow back perfectly, bone by bone, muscle by muscle—without scars. For humans, this is pure science fiction. But for the Mexican salamander known as the axolotl (Ambystoma mexicanum), this is everyday reality. These fascinating creatures have captivated scientists for decades, now standing at the frontier of regenerative medicine.
A breakthrough study by researchers at Northeastern University, published recently in Nature Communications, highlights a molecule called retinoic acid—a derivative of vitamin A and a common ingredient in acne medications like isotretinoin. This study reveals how retinoic acid guides the cellular "GPS" within axolotls during limb regrowth, orchestrating the precise rebuilding of upper arms, forearms, hands, or digits (nationalgeographic.com).
Understanding this process brings us one step closer to teaching the human body to regenerate complex tissues again. This article dives deep (over 1,300 words!) into how axolotls regrow limbs, the role of retinoic acid, and what this means for future therapies in humans.
1. Why Axolotls? The Superheroes of Regeneration
Axolotls are critically endangered in the wild around Mexico City, yet thrive in labs as living research tools (washingtonpost.com). Their key features include:
-
Regrowing entire limbs, including bones, nerves, muscles, skin—restored perfectly, not like scar tissue.
-
The ability to regenerate heart, lung, and even brain tissues—capable of regrowing spinal cord segments too (smithsonianmag.com, nationalgeographic.com, mdpi.com).
-
Unique juvenile traits in adulthood: external gills, finned tail, and a perpetual smile.
This extraordinary regenerative ability makes the axolotl an ideal model for unlocking the secrets of positional memory—how cells know what and where to grow.
2. The Role of Retinoic Acid in Regeneration
What is Retinoic Acid?
-
A vitamin A metabolite, also known as a retinoid.
-
Present in human biology—important during embryonic development for head‑to‑tail patterning.
-
Used in skincare (e.g., acne treatments).
-
Axolotls use it as a positional cue during limb repair
How It Works in Axolotl Limbs
Northeastern’s biologist James Monaghan and his team performed detailed experiments:
-
Flashy fluorescence: Axolotls were genetically modified to glow green wherever retinoic acid was active, enabling real-time tracking (washingtonpost.com).
-
Gradient discovery: Higher RA levels near shoulders, lower in hands. Inhibitor CYP26B1, an enzyme that breaks down RA, is more active distally—creating the gradient (news.northeastern.edu).
-
Dose-dependent growth:
-
Extra RA injected: A forearm trimmed halfway could regrow an entire upper arm—"Frankenstein limbs" (dnyuz.com).
-
Enzyme blocker (CYP26B1 inhibitor, e.g., talarozole): The gradient steadied; depending on level, limbs regrew incorrectly—hand became arm, or repeated segments appeared (news-medical.net).
-
-
Gene expression mapping:
-
Meis1/Meis2 markers: Activated when RA high (proximal identity).
-
Hoxa13: Activated when RA low (distal identity).
-
Shox gene: A key driver. Without Shox, arms were short—hand without enough upper-arm structure (cos.northeastern.edu, news-medical.net).
-
This precise molecular choreography shows how axolotl cells gain positional awareness, ensuring correct limb regrowth.
3. The Enzyme CYP26B1: The On/Off Switch
A key finding is the role of the enzyme CYP26B1, which:
-
Degrades retinoic acid, shaping the RA gradient.
-
Higher near hands → less RA = distal regrowth.
-
Lower near shoulders → more RA = proximal regrowth (news.northeastern.edu).
Blocking CYP26B1 leads to excessive RA levels, shifting the growth program toward more proximal (upper-limb) identity—even when only a forearm or hand is missing .
Thus, CYP26B1 is the molecular switch, helping cells remember where they are on the limb and what structures to rebuild. Without it, the regrowth program misfires.
4. The Genetic Blueprint: Shox, Meis, and Hoxa Genes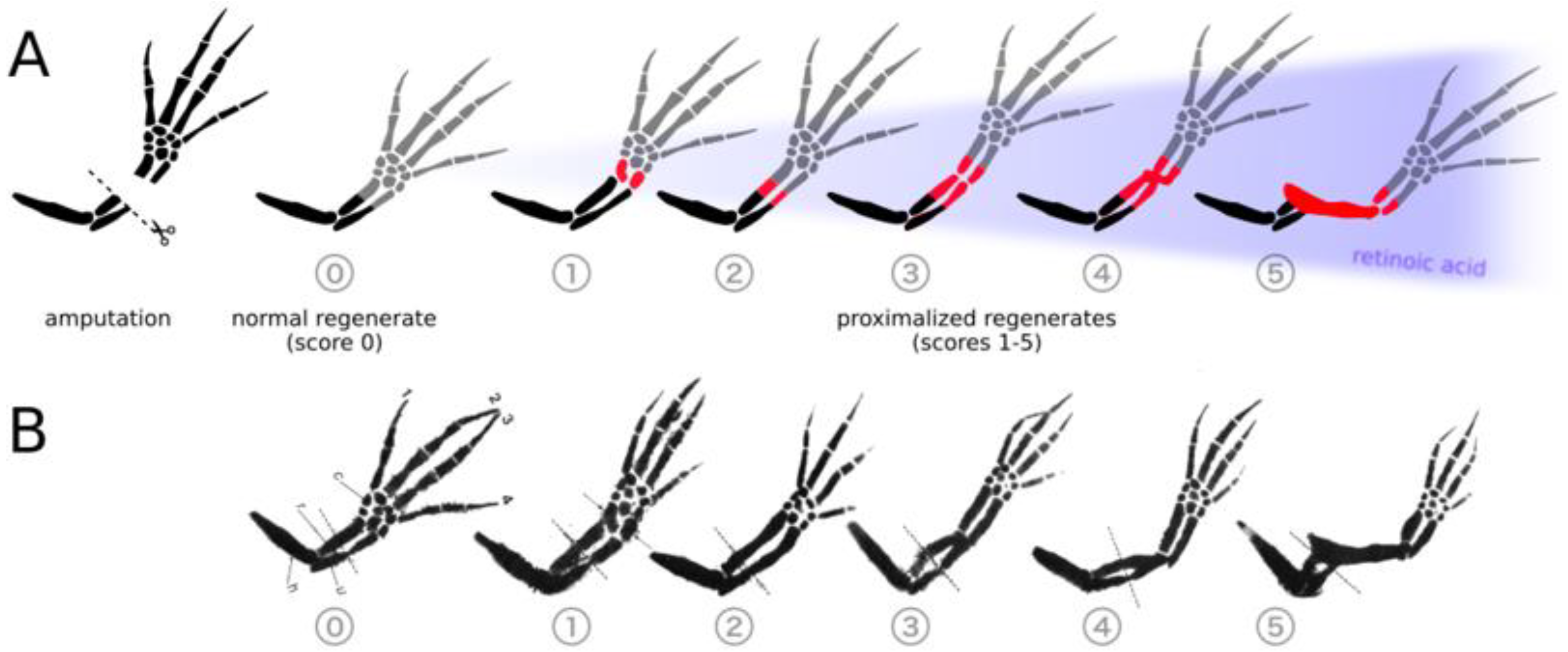
Regeneration involves reactivating embryonic genes that direct limb formation. Among these are:
-
Meis1/Meis2: Proximal identity (shoulder/upper arm).
-
Hoxa13: Distal identity (hand/digits).
-
Shox: Critical in executing RA’s instruction. Without it, axolotls regrow hands but not full arms (smithsonianmag.com).
By using CRISPR-Cas9, Monaghan’s team turned off Shox to see its effects during RA-enhanced regeneration:
-
Without Shox: limbs regrew, but arms were too short or lacked bone structure.
-
With RA + Shox functional: limbs duplicated or extended appropriately (news-medical.net, mdpi.com).
This reveals Shox as the executioner gene, allowing the RA positional signal to become real tissue.
5. Can This be Applied to Humans?
The Good News:
-
Humans share retinoic acid pathways, CYP26 enzymes, Meis, Hoxa, Shox genes
-
Human embryos use RA to form body plans, suggesting deep homology.
-
We already produce and respond to RA, especially in early development.
The Challenge:
-
Adult human cells don’t dedifferentiate — they form scar tissue instead of blastema.
-
We lack the natural ability to “turn off” adult identity and re-enter embryonic programs.
-
The complexity of limb structure—bones, nerves, blood vessels—requires precise orchestration.
But MIT and Northeastern teams believe it's not impossible. If we can:
-
Trigger blastema formation (dedifferentiation).
-
Control RA gradients or block RA breakdown in injured areas.
-
Activate genes like Shox, Meis, Hoxa at the right time.
—then we might coax human limbs to regrow—over years, not weeks
6. Beyond Limbs: Wider Implications in Medicine
Studying axolotls may lead to breakthroughs beyond amputations:
-
Spinal cord repair, heart regeneration, lung tissue regrowth—all observed in axolotls (washingtonpost.com).
-
Tissue engineering: RA gradients might help scaffold cells into limbs in the lab.
-
Scar reduction: Temporarily manipulating CYP26B1 could reduce scarring in humans.
-
Cancer insights: Regeneration and uncontrolled cell growth share molecular circuits.
This leads to promising tools like gene therapy, small-molecule regulators, and stem-cell treatments targeting RA pathways.
7. How the Experiments Were Done
Key techniques employed:
-
Fluorescent RA reporters: Genetically modified axolotls glow where RA activity is high (dnyuz.com).
-
Gradient mapping: Tissue-level RA measurement through gene expression.
-
Drug manipulation: Talarozole (CYP26B1 inhibitor) and RA analogs.
-
Molecular assays: qRT‑PCR, scRNA‑seq, HCR‑FISH revealed gene expression.
-
Genetic editing: CRISPR used to knock out Shox gene (news-medical.net).
These powerful techniques allowed precise dissection of the regeneration signaling cascade.
8. Why This Study Matters—Summary
-
Identifies RA gradient as positional cue in limb regrowth.
-
Highlights CYP26B1 as master regulator of that gradient.
-
Confirms downstream gene network: Meis, Hoxa13, and especially Shox.
-
Demonstrates limb patterning can be manipulated—excess RA or blocking breakdown alters limb identity.
-
Suggests new therapeutic strategies for human regenerative medicine.
Northeastern’s Prof. Monaghan stated:
“Understanding how a limb knows what to grow back…was a long-standing mystery…and this study gives us insight at the molecular level”
9. What’s Next in Regeneration Research?
Ongoing & future directions:
-
Fine‑tuning RA signals: More selective CYP26 inhibitors, timing and delivery methods.
-
Shox pathway exploration: Mapping downstream targets and partners.
-
Human cell studies: Testing if RA gradients prompt blastema‑like responses in human fibroblasts or stem cells.
-
Gene editing & therapy: Animal trials guiding translation to mammals.
-
Broad regenerative targets: Heart, spinal cord, facial tissue—RA is a universal envelope drive.
Ethical and practical steps:
-
Incremental trials in animals with regenerative potential (lizards, frogs).
-
Safety checks: RA dysregulation can cause developmental abnormalities.
-
Continued basic research to avoid unintended side effects.
Conclusion: A Vision for Regenerative Futures
The axolotl may appear modest and even bizarre, yet it holds the blueprint for unlocking regenerative power that humans lost millions of years ago. This groundbreaking study cracks open a piece of that mystery by revealing:
-
Retinoic acid gradient = cellular GPS.
-
CYP26B1 = balance keeper.
-
Shox and co. = builders.
From here, scientists dream of teaching human cells to regrow tissues, not just patch wounds.
Yes, human limb regeneration is still distant. But with each discovery—from glowing salamanders to CRISPR-tested genes—we move from science fiction to science fact. And as Prof. Monaghan puts it:
“We all made these limbs when we were embryos…^the question is how to turn those programs back on” (washingtonpost.com).
The journey is long, but the roadmap is clearer than ever.
Quick FAQs
Q: Is human limb regeneration possible?
A: Not yet. But we share essential molecules—RA, CYP26B1, Shox genes—with axolotls. The path is plausible .
Q: What does RA do?
A: Acts like a GPS: higher near shoulders → arm growth; lower near hand → digits form (washingtonpost.com).
Q: Why block CYP26B1?
A: It breaks down RA. Blocking it raises RA levels, confusing the cells into rebuilding incorrectly .
Q: What role does Shox play?
A: Executes RA’s instructions. Without it, arms are incomplete or malformed .
Q: Could RA treatments harm humans?
A: High RA often causes birth defects. Safety and dosage control will be critical in regenerative trials (ktvz.com).
Closing Thoughts
From glowing axolotls in fluorescent labs to precision gene editing, this research represents a leap forward in understanding how nature rebuilds limbs. It's a powerful reminder: what once seemed like sci‑fi—regrowing human limbs—may one day be real. And it all starts with a humble salamander that refused to lose its limbs.
Open Your Mind !!!
Source: Nationalgeographic



Comments
Post a Comment